
DRO
Digital・Decentralized・Data
Research Organization
Revolutionizing
Clinical Trials:
Faster Results,
Lower Costs!
Formation of business infrastructure through the construction of clinical trial/clinical research operation management systems
Building a case accumulation foundation through a new clinical trial network co-created with partner sites originating from clinical trial medical institutions
Support for highly accurate feasibility studies through patient data linkage
Utilization of electronic medical record data (structured data integration/unstructured data) and RWD
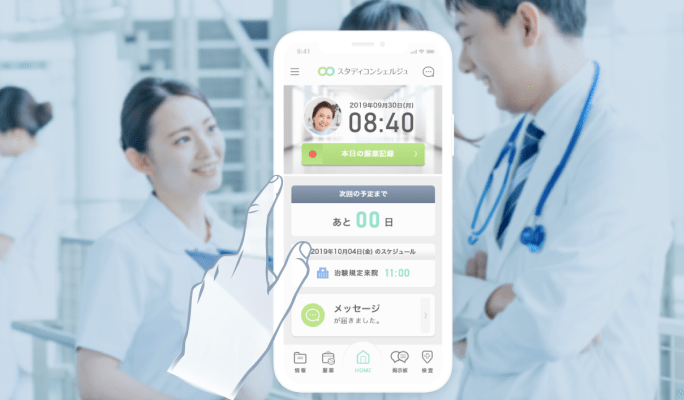
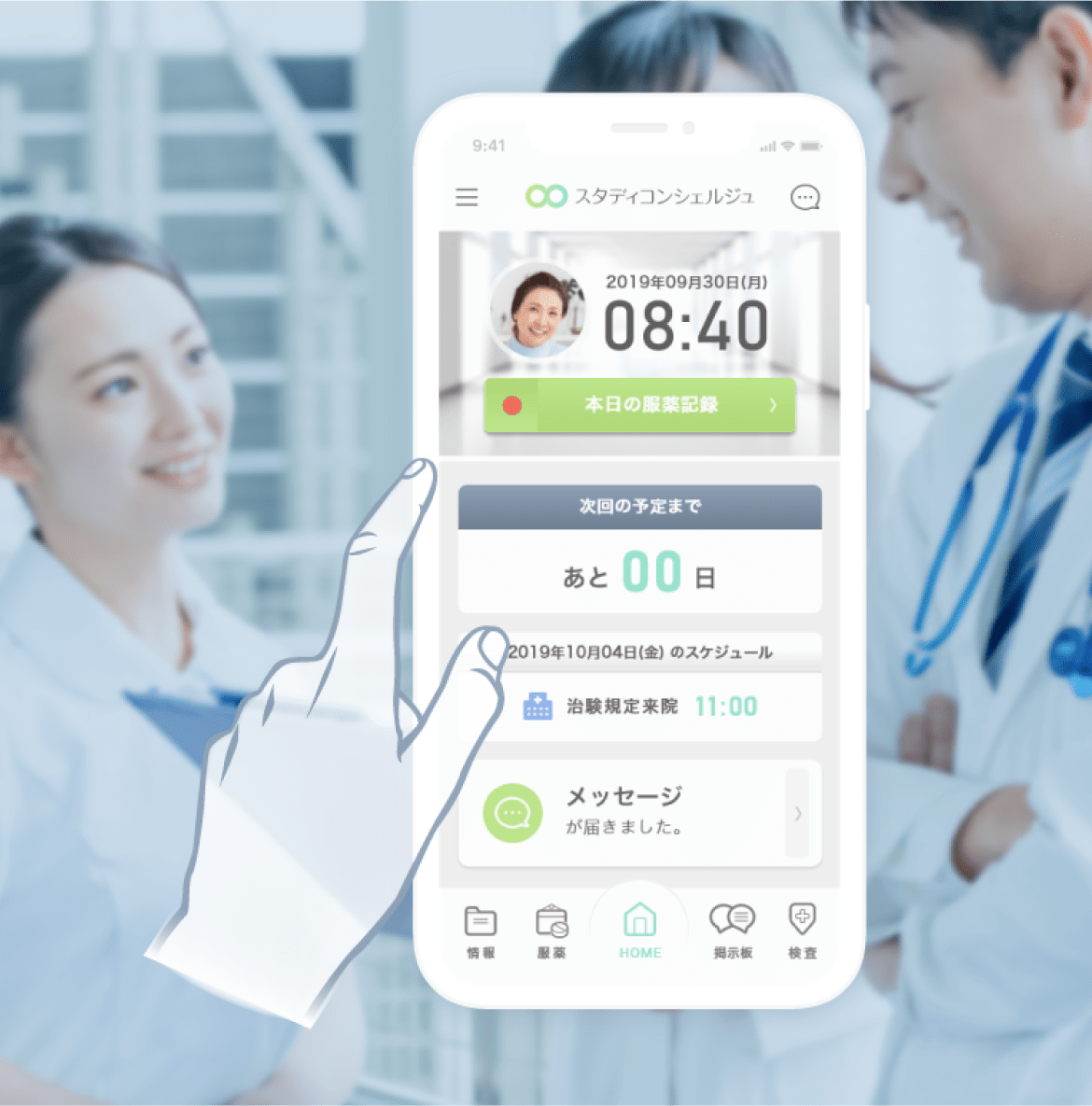
DCT business management platform
eRecruit / eConsent / Online medical consultation / Delivery of investigational drugs / Wearable devices / Sample collection / Home nursing / Partner site operations / Cost management
*AI and data utilization will be implemented in the future.


Service
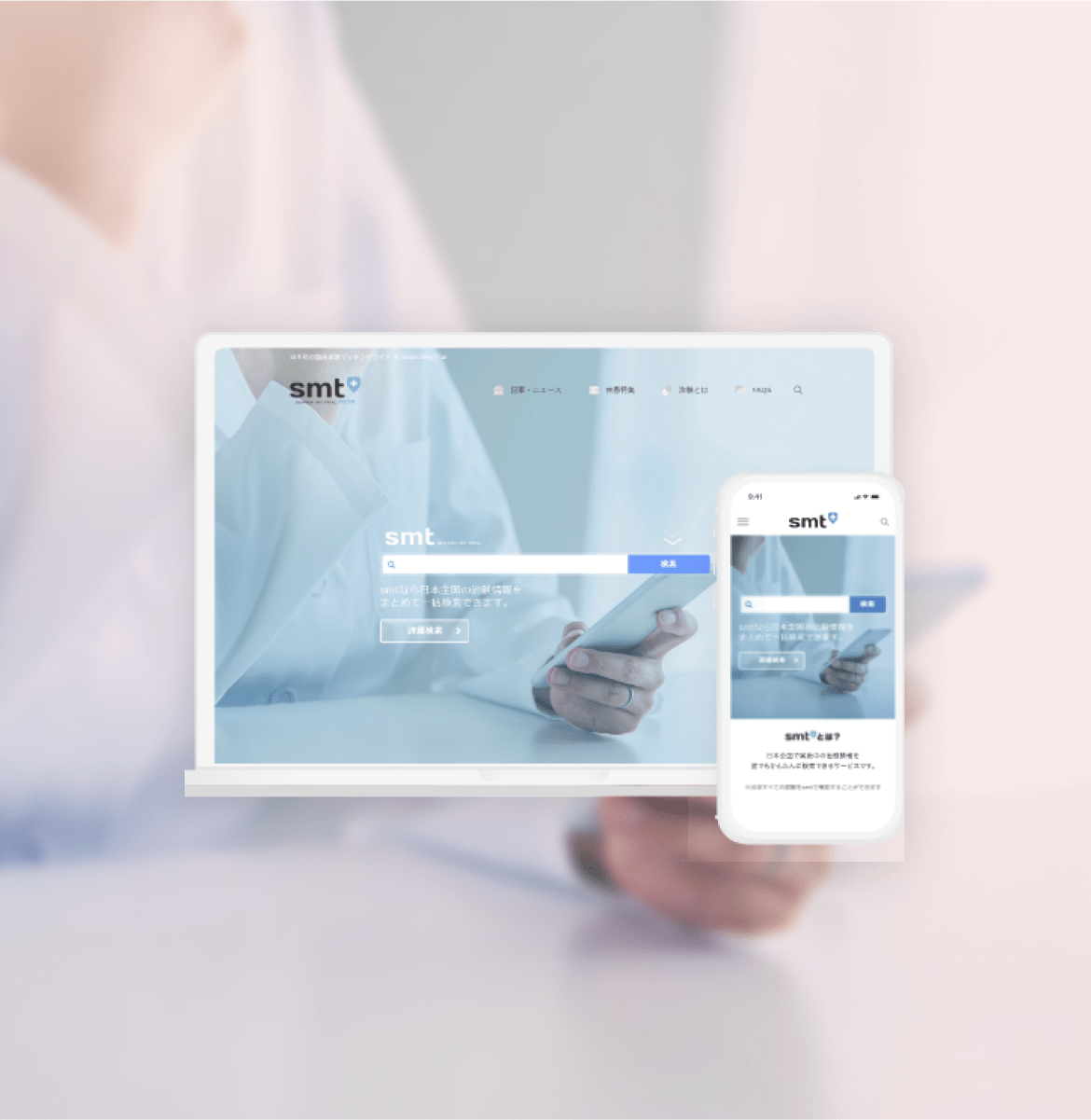
Introducing StudyWorks Platform:
A SaaS platform solving clinical trial and research challenges
For clinical trials
“StudyWorks Platform”
Press

Buzzreach supports the revitalization of specific clinical research and investigator-initiated clinical trials in Japan. Launches project management support service utilizing StudyWorks® and specialized personnel.

Corporate exhibition booth and luncheon seminar held at the 17th Annual General Meeting of the Japanese Society of Clinical Trials

Strategic partnership with eCOA/DCT vendor Xincere to attract more clinical trials to Japan

Dr. Yasuo Takeda, Chairman of the Japanese Society of Hospital Pharmacists, has been appointed as an advisor to our company.

New Year’s Greetings

Notice of New Year’s holiday closure and thanks for the year

COO Aoyagi spoke at Chinatriaals I7 held in Shanghai.

Lecture report by the “StudyWorks®” Product Development Department Manager released – The challenge of developing a DCT platform in collaboration with clinical trial sites –

Career
A place where ideas thrive and innovation happens daily.
Come join us and be a part of shaping the future!